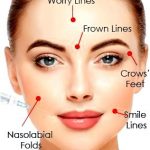
wrinkles on your face and folds.1,2
wrinkles on your face and folds.1,2
The scientifically-advanced gels are produced with XpresHAn Technology, creating gels that provide a variety of versatility and support3,4,5 for varied patient needs. Restylane Refyne and Restylane Defyne happen to be proven to keep effectiveness to treat laugh lines for approximately 12 several weeks.1,2
"Despite current approved options, a lot of my people are still searching for various methods to treat their laugh lines," stated Boca Raton-based oculoplastic surgeon and Restylane Refyne clinical investigator Steven Fagien, M.D. "These new items are flexible and are made to meet different patient needs. I’m excited to provide these next-generation hyaluronic acidity (HA) dermal fillers within my practice."
"Restylane Refyne and Restylane Defyne would be the latest Food and drug administration-approved advancements in HA dermal fillers and align with Galderma’s mission to help those achieve natural-searching results through treatments having a lengthy-standing good reputation for proven safety and effectiveness," stated Kelly Huang, Ph.D., VP & gm from the U.S. Aesthetic and Corrective business of Galderma. "We had an chance to deal with a typical concern for patients who haven’t yet attempted a dermal filler by designing gels that offer natural-searching results. Using these new brands, the Restylane group of products now represents the largest offering of HA dermal fillers within the U.S."
The Food and drug administration approval took it’s origin from two pivotal, double-blinded, randomized, active-controlled Phase 3 studies investigating Restylane Refyne and Restylane Defyne (involving 171 and 162 subjects, correspondingly) to judge their safety and effectiveness. Both in studies, Restylane Refyne and Restylane Defyne met the studies’ endpoints, with products showing a clinically significant improvement in wrinkle severity for approximately 12 several weeks in nearly all patients.
Study investigators used the Wrinkle Severity Rating Scale (WSRS), a validated 5-point way of measuring the dimensions and depth from the wrinkles, with grade 1 understood to be lack of wrinkles and grade 5 as very deep and lengthy wrinkles. Investigators reported that 79% of Restylane Refyne subjects and 77% of Restylane Defyne subjects had a minimum of single-grade step up from the WSRS after 6 days.
Subjects also performed self-assessments (SSA) of wrinkle severity, with many subjects reporting a minimum of single-grade improvement in SSA scores with Restylane Refyne with Restylane Defyne after 6 days.1,2
"A lot of my people are interested to discover the most recent products that will help them achieve natural-searching results, but they are oftentimes unsure about beginning dermal fillers," stated North Park-based board-certified skin doctor Mitch Goldman, M.D. "The development of these next-generation HA dermal fillers with XpresHAn Technology can change my patients’ thoughts about fillers. Restylane Refyne and Restylane Defyne provide choices for patients who wish to make certain they are able to achieve natural-searching results, that is a key need my patients express every single day."
After initial treatment, injection site responses (redness, swelling, bruising, lump/bump formation, discomfort/tenderness) were predominantly mild or moderate in intensity, temporary (typically having a time period of one or two days), and other alike for that Restylane products.
__________________________________________________
References:
1 Galderma. Restylane Refyne Instructions to be used. 2016.
2 Galderma. Restylane Defyne Instructions to be used. 2016.
3 A pivotal USA randomized evaluator-blinded, active-controlled, multi-center, split-face comparison study of Emervel Deep Lidocaine versus Juvederm Ultra Also in treating moderate to severe wrinkles on your face and folds [Phase 3 Support Study: RD.06.CIR.18159 CSR Emervel Deep, phase 3 versus Juvederm Ultra Plus, 79w]
4 A pivotal USA randomized evaluator-blinded, active-controlled, multi-center, split-face comparison study of Emervel Classic [Phase 3 Support Study: RD.06.CIR.18156 CSR Emervel Classic, phase 3 versus Juvederm, 76w]
Lidocaine versus Juvederm Ultra in treating moderate to severe wrinkles on your face and folds
5 Look at amplitude sweep mix-over point being an index of versatility [MA-32418 Final Versatility Report], October 4, 2016.
(Source: PR Newswire)
Resourse: https://rdmag.com/news/2016/12/
 Proskin Clinics
Proskin Clinics